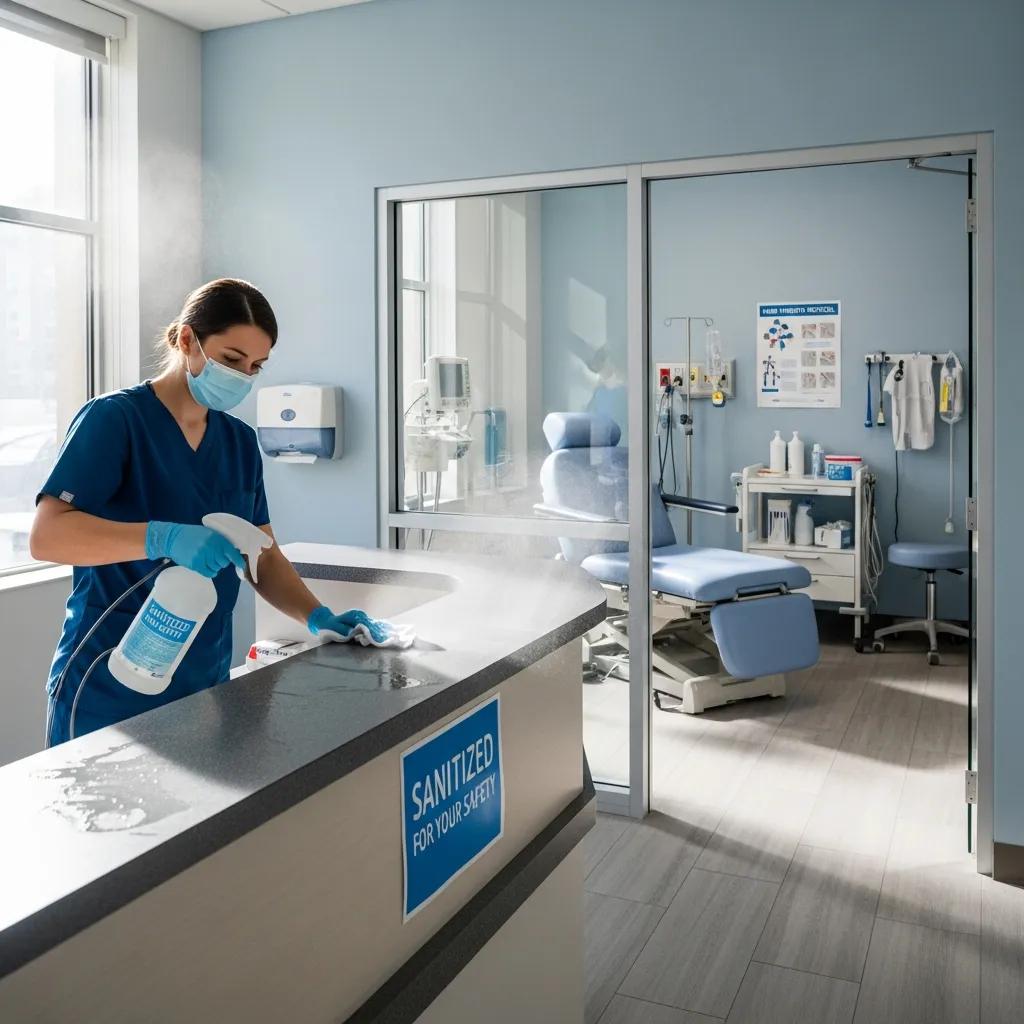
Specialized Medical Office Cleaning in Ann Arbor, MI — Clinical‑grade sanitation you can trust
Medical office cleaning requires clinical‑grade sanitation that removes pathogens, protects patients and staff, and helps facilities meet regulatory expectations. We use EPA‑ and hospital‑grade disinfectants, targeted surface and air treatments, and documented protocols to lower infection risk and keep Ann Arbor outpatient clinics safe and compliant. This guide explains how clinic sanitation differs from standard commercial cleaning, what services and frequencies work best, and how CDC, OSHA, and HIPAA influence daily operations. You’ll find practical service options, infection‑control requirements, dental and specialty clinic procedures, and the steps to arrange a tailored cleaning program. It’s written for clinic managers, dental leads, facility directors, and property managers who need dependable medical cleaning in Ann Arbor.
Why choose McCoy Maintenance for medical office cleaning in Ann Arbor?
Picking a medical cleaning partner means looking for clinical experience, trained staff, and methods built around infection prevention. Providers with written protocols, hospital‑grade products, and supervised training reduce exposure risks and help outpatient facilities meet regulatory standards. Below are the credibility points clinic managers should look for when vetting local specialists — and the reasons McCoy Maintenance aligns with those expectations.
- Locally owned and operated — long‑term community presence and consistent service.
- Custom cleaning plans backed by a 100% satisfaction guarantee to ensure the scope fits your practice.
- Trained, background‑checked, and insured staff, supervised by a certified Master Cleaner.
- Use of hospital‑grade disinfectants and electrostatic application when appropriate for clinical areas.
These credentials summarize local credibility and operational readiness, and they lead into our experience, certifications, and how we structure custom plans for different clinic types.
What experience and certifications support our medical cleaning expertise?
Experience and certification show cleaning teams understand clinical risk zones and the techniques that reduce transmission in outpatient settings. In Ann Arbor, that looks like defined leadership, supervisory structures that enforce infection‑control SOPs, consistent PPE use, and documented quality checks. Training typically covers environmental disinfection, bloodborne pathogen awareness, safe handling of clinical waste, and HIPAA‑aware behaviors when working near patient records. A certified Master Cleaner provides technical oversight and ongoing staff education to keep performance consistent and measurable for healthcare janitorial services.
Our routine quality assurance includes checklists, supervisory walkthroughs, and documented corrective actions. Once staff competency is established, we tailor frequencies and methods to a facility’s patient volume and risk profile so that training and protocols translate into reliable, day‑to‑day infection control and patient safety outcomes.
How do customized cleaning plans meet unique Ann Arbor medical facility needs?

Custom plans start with an on‑site assessment to identify high‑risk zones, patient flow, and scheduling constraints specific to your practice. We create a prioritized task matrix that allocates daily high‑touch disinfection, mid‑day cleaning for busy reception areas, and terminal cleaning after procedures when required. Each plan documents frequencies, PPE levels, and reporting routines so facility managers have clear expectations and audit trails. Small specialty clinics may emphasize operatory turnover and instrument‑adjacent surfaces, while multi‑specialty practices benefit from phased scheduling and dedicated night teams to minimize patient disruption.
Regular review cycles and QA reporting let us adjust as patient volume fluctuates or during seasonal infection surges, keeping the cleaning plan aligned with your operational needs. That flexibility connects directly to the services and methods used in clinical sanitation, described next.
What comprehensive medical cleaning services are offered in Ann Arbor?
Comprehensive medical cleaning blends routine janitorial work with clinical‑grade disinfection, targeted deep cleaning, and floor and textile maintenance to protect patient safety and facility appearance. Typical components include daily high‑touch surface disinfection, electrostatic spraying for room‑level pathogen reduction, exam‑room turnover cleaning, and scheduled floor care (stripping, sealing, carpet extraction) based on flooring. These services support regulatory compliance and reduce fomite transmission risks, and they can be scaled to match patient volume and specialty. The table below helps facility managers compare common disinfection approaches and choose what fits their clinic.
Different disinfection methods have specific use cases and recommended frequencies in medical settings.
| Disinfection Method | Typical Application | Recommended Frequency |
|---|---|---|
| Daily manual surface disinfection (CDC‑listed products) | High‑touch surfaces — door handles, check‑in counters, exam tables | Daily and between patients for high‑touch items |
| Electrostatic spraying | Wide surface coverage in rooms and waiting areas | Weekly or after exposure events; used as an adjunct to manual cleaning |
| Fogging (hospital‑grade) | Terminal or outbreak‑level deep disinfection of unoccupied spaces | As needed for terminal cleaning or post‑exposure |
| Carpet extraction | Deep‑clean carpets to remove soils and pathogens | Quarterly or after construction/renovation |
How do hospital‑grade disinfectants and fogging enhance clinic sanitation?
Hospital‑grade disinfectants are EPA‑registered products formulated and tested to achieve specific reductions in healthcare‑associated pathogens when used per label instructions. Effective sanitation depends on proper contact time (dwell time) and thorough surface coverage. Fogging and electrostatic spraying help reach hard‑to‑clean areas and can reduce manual labor, but they don’t replace manual cleaning where visible soil remains; fogging is most useful for terminal cleans or after known exposures. Safety protocols require rooms to be vacated during fogging, operators to use appropriate PPE, and strict adherence to manufacturer re‑entry times to protect patients and staff.
Knowing when to use manual disinfection versus adjunct technologies lets facility managers pick the right mix for exam rooms, procedure suites, and communal spaces. That choice informs janitorial and floor‑care protocols needed to keep your environment clean and safe.
What janitorial and floor maintenance services support healthcare facilities?
Routine janitorial work in medical offices focuses on high‑touch surface disinfection, regulated waste handling, restroom sanitation, and supply restocking to support infection control. Daily tasks usually include disinfecting vestibules, reception counters, exam tables, and staff areas, plus coordinated trash and medical‑waste procedures that meet OSHA bloodborne pathogen guidance. Floor care — scheduled stripping and waxing for hard surfaces and extraction for carpets — prevents soil buildup that can harbor microbes and keeps surfaces cleanable and slip‑resistant. Frequencies scale with patient traffic: daily for busy clinics, weekly for moderate traffic, and monthly or quarterly for restorative floor work.
Good floor and textile maintenance lowers airborne particulates and extends material life, while rigorous restroom protocols reduce pathogen reservoirs and odors. Together, these janitorial practices form a continuum from hourly touchpoint management to periodic deep cleaning and maintenance, directly supporting regulatory compliance.
Elevating Medical Facility Cleanliness in Ann Arbor

Infection control and regulatory compliance set the operational and documentation standards medical cleaning teams must follow. Agencies like the CDC and OSHA translate into practical expectations: EPA‑registered disinfectants, PPE for bloodborne pathogen exposures, training records, and documented cleaning logs. HIPAA adds privacy requirements that affect how cleaners handle paper records and electronic devices. Together, these frameworks demand procedural rigor, competent staff, and transparent reporting to show a facility is clean, safe, and compliant with public‑health and occupational‑safety rules.
Use the short compliance checklist below to evaluate potential cleaning partners. It helps facility managers confirm that cleaning operations support infection prevention goals and meet regulatory requirements.
- Product and procedure verification: Confirm disinfectants are EPA‑registered and used per labeled dwell times.
- PPE and bloodborne pathogen controls: Ensure staff use appropriate PPE and follow sharps/waste protocols.
- Training and documentation: Maintain training records, cleaning logs, and incident reports for audits.
- Privacy and access controls: Verify procedures for protecting PHI and cleaning restricted areas.
These core elements map directly to guideline summaries and operational requirements shown in the table below, linking authoritative sources to on‑the‑ground cleaning practices.
| Guideline Source | Guideline Focus | Practical Requirement |
|---|---|---|
| CDC environmental infection control | Surface disinfection and high‑touch cleaning | Use EPA‑registered disinfectants; document frequency and methods |
| OSHA bloodborne pathogens | Exposure control and PPE | Provide training, PPE, and waste handling procedures |
| HIPAA privacy rule | Protection of PHI in physical spaces | Establish supervised access and staff confidentiality agreements |
What CDC and OSHA guidelines govern medical office cleaning protocols?
CDC guidance for outpatient settings focuses on targeted cleaning of high‑touch surfaces, choosing appropriate disinfectants, and matching cleaning frequency to patient flow and risk. Recommendations prioritize manual cleaning supplemented by technologies when appropriate, always emphasizing correct product contact times and visible cleanliness before disinfection. OSHA standards center on exposure control plans for bloodborne pathogens, PPE requirements, employee training, and procedures for regulated waste and sharps. For cleaning teams, OSHA requires documented training, supplied PPE, and clear protocols to reduce occupational exposure when cleaning blood or body fluids.
To put these guidelines into practice, clinics should create SOPs that spell out products, task frequencies, PPE for specific tasks, and staff training schedules. Consistent documentation — training logs, body‑fluid cleanup reports, and daily cleaning checklists — forms the audit trail needed to demonstrate compliance and support continuous improvement.
How is HIPAA compliance maintained during cleaning services?
Protecting HIPAA during cleaning means having policies that prevent accidental exposure of protected health information. Practical steps include locking or removing patient charts before cleaning, avoiding vacuuming or dusting near unsecured records, and training cleaning staff on confidentiality and supervised access. Contracts and confidentiality agreements should cover cleaning personnel, and protocols should require electronic devices containing PHI to be powered off and secured during service. Supervisory spot checks verify cleaners aren’t photographing or handling patient information and that access procedures are followed.
Coordination between facility leadership and cleaning providers ensures schedules and access controls support patient privacy, and that any incidents involving PHI are reported and remediated per facility policy. These behaviors work alongside disinfecting and maintenance activities to protect patients and staff.
What are the specialized cleaning requirements for dental and clinic facilities in Ann Arbor?
Dental and specialty clinics demand tighter cleaning controls because of procedural aerosols, proximity to instruments, and sterilization workflows that must be synchronized. Operatories, sterilization rooms, and recovery areas each play distinct roles: operatories require quick between‑patient surface disinfection, sterilization adjacencies need dust control and restricted access, and waiting areas benefit from regular high‑touch cleaning. Cleaning teams must coordinate with clinical staff to avoid disrupting sterilization checks and to ensure environmental cleaning supports instrument processing and procedural workflows.
Timing—such as end‑of‑day terminal cleaning after sterilization cycles—prevents cross‑contamination and keeps the clinical environment reliable for patient care. The next section explains typical operatory sanitation steps and frequencies for dental offices.
How are dental offices sanitized to meet industry standards?
Dental sanitation focuses on immediate surface disinfection after each patient, with extra attention to dental chairs, light handles, counters, and chairside equipment. Disposable barriers and surface disinfectants help limit contamination; manual cleaning precedes disinfection when visible soil is present. End‑of‑day deep cleaning — including floors and equipment adjacencies — removes settled particulates and prepares operatories for the next day. Coordination with sterilization room workflows ensures instrument handling and environmental cleaning don’t overlap in a way that risks exposure or disrupts sterilization cycles.
These operatory practices reduce cross‑contamination and align environmental cleaning with instrument reprocessing, creating a continuous protective buffer around procedural areas.
What disinfection practices are essential for Ann Arbor clinics?
Essential disinfection practices emphasize identifying high‑touch points, choosing the right disinfectant class, and following dwell times that ensure pathogen inactivation. High‑touch items include equipment controls, door handles, check‑in surfaces, chair arms, and restroom fixtures; these typically require daily disinfection and, in busy clinics, cleaning between patients. Recommended products are EPA‑registered hospital disinfectants effective against enveloped and non‑enveloped viruses — used with correct dilution, storage, and contact times. Indoor air quality measures like HEPA filtration and adjunct fogging can supplement surface cleaning in settings with aerosol risk but should not replace manual disinfection.
Prioritizing touchpoints and pairing manual cleaning with appropriate adjunct technologies yields the best reduction in fomite transmission while supporting patient and staff safety. The following section outlines measurable benefits and return on investment from professional medical cleaning.
How does professional medical cleaning benefit Ann Arbor healthcare facilities?
Professional medical cleaning delivers measurable benefits: fewer infections, better patient‑safety metrics, and a polished professional image that builds patient confidence and referrals. By systematically removing pathogens and keeping environments cleanable, clinics reduce opportunities for healthcare‑associated infections and lower staff absenteeism from transmissible illnesses. These operational gains support continuity of care, reduce liability risk, and contribute to higher patient satisfaction by presenting a visibly well‑maintained facility.
Documented cleaning protocols and QA reporting give facility managers KPIs — such as reduced absenteeism and improved patient feedback — to track the clinical and financial ROI of enhanced sanitation. That link between cleaning practice and outcomes highlights cleaning’s role in infection reduction, detailed next.
What role does cleaning play in reducing infection risks and enhancing patient safety?
Cleaning reduces infection risk by removing soils and applying disinfectants that inactivate pathogens on surfaces that act as fomites, interrupting transmission pathways in outpatient settings. Regular disinfection of high‑touch points, plus targeted deep cleans after suspected exposures, lowers environmental bioburden and the chance that pathogens reach vulnerable patients or staff. Monitoring metrics such as infection incident reports, staff sick days, and environmental ATP or swab testing can quantify program effectiveness and guide improvements. Integrating cleaning data into infection‑prevention plans helps facility managers prioritize interventions and show the safety gains of structured sanitation programs.
These measurable impacts also improve staff well‑being and organizational reputation, described next.
How does specialized cleaning improve staff well‑being and facility reputation?
Specialized cleaning reduces occupational exposure, lowers illness‑related absenteeism, and creates a workplace perceived as safe and professionally cared for. Cleaner facilities boost morale and retention, letting staff focus on clinical work when environmental safety is managed reliably. Patients notice cleanliness and report higher satisfaction when facilities look well maintained, which supports referrals and community trust. Tracking KPIs — fewer sick days, higher patient satisfaction scores, and fewer incidents — helps quantify the indirect financial and operational returns of professional cleaning.
Those benefits make a strong case for clinics to adopt tailored cleaning programs. The next section explains how to request services and onboard a provider.
How can Ann Arbor medical offices request customized cleaning services?
Requesting customized cleaning follows a straightforward process: initial contact and information gathering, site assessment, proposal and scope development, then onboarding with a pilot phase before full implementation. Clear details about patient volumes, peak hours, procedure types, and restricted areas speed accurate scoping and pricing. Expect a written proposal outlining frequencies, products, PPE, QA reporting, and a trial period to validate performance. Collaboration is key so cleaning schedules complement clinical workflows and preserve HIPAA safeguards during service delivery.
Below is a concise process to help clinic managers move from assessment to implemented service quickly and transparently.
- Request an initial consultation and provide a brief practice profile and preferred contact details.
- Host a site visit for risk assessment, scope identification, and high‑touch mapping.
- Receive a written proposal with recommended frequencies, disinfection methods, and QA metrics.
- Begin a pilot period with documented checklists and review meetings to finalize the full schedule.
What is the process for scheduling a free consultation with McCoy Maintenance?
Scheduling begins with a short outreach step where clinics share practice type, size, and service hours for initial scoping, followed by a site visit to document risk areas and patient flow. During the visit a qualified representative notes high‑touch surfaces, floor types, and any special requirements like sterilization room adjacency or privacy constraints. After assessment, McCoy Maintenance delivers a tailored proposal detailing recommended frequencies, disinfectant methods (manual, electrostatic, or fogging where appropriate), and QA reporting options. A brief pilot period is used to fine‑tune the plan and confirm schedules and outcomes meet clinic expectations.
This transparent approach keeps recommendations evidence‑based and aligned with operational realities so clinics can adopt cleaning programs that balance infection control, patient safety, and workflow continuity.
How are service plans tailored to meet specific medical facility needs?
We tailor service plans by applying risk‑based tasking that adjusts frequencies to patient throughput, procedural risk, and facility layout, with extra customization for dental operatories, urgent care triage areas, or other specialty needs. Customized SOPs define PPE levels for specific tasks, provide cleaning checklists for each zone, and schedule QA inspections to verify adherence. Pilot periods let both parties collect performance data and refine schedules, while ongoing reporting supports incremental adjustments for seasonal changes or operational shifts. This iterative tailoring keeps the cleaning plan effective and responsive to evolving clinical demands.
Combining risk assessment, SOP customization, and measurable QA cycles helps clinics sustain cleaning regimens that support infection prevention, regulatory compliance, and a safer environment for patients and staff.
Frequently asked questions
What types of disinfectants are used in medical office cleaning?
We use EPA‑registered, hospital‑grade disinfectants proven to eliminate common healthcare pathogens when used per label directions. Product choice depends on the task — some are formulated for high‑touch surfaces, others for broader applications like fogging. Correct use, including required contact times, is essential to achieve the expected level of disinfection.
How often should medical offices undergo deep cleaning?
Deep‑clean frequency depends on patient volume, services offered, and infection‑control needs. High‑traffic areas may need weekly or bi‑weekly deep cleaning, while surgical suites or dental operatories might require daily terminal cleaning after procedures. During outbreaks or higher community transmission, increased deep‑cleaning cadence may be necessary. Regular assessments help determine the optimal schedule.
What training do cleaning staff receive for medical office sanitation?
Our cleaning staff complete comprehensive training covering PPE use, bloodborne pathogen controls, regulated waste handling, surface disinfection techniques, and HIPAA‑aware conduct. Ongoing education keeps teams current on best practices and new technologies, ensuring consistent, safe service in clinical environments.
How can medical offices ensure compliance with HIPAA during cleaning?
Protecting HIPAA during cleaning requires secure handling of records and controlled access. Practices should secure or remove patient charts, limit cleaning near unsecured devices, and train cleaning staff on confidentiality and supervised access. Regular audits and supervisory checks help ensure cleaning activities do not compromise patient privacy.
What are the benefits of using specialized cleaning services for medical offices?
Specialized cleaning delivers better infection control, improved patient safety, and a professional appearance that builds patient trust. Tailored protocols ensure high‑touch surfaces are regularly disinfected and cleaning aligns with regulatory standards. Benefits also include reduced staff absenteeism, improved patient satisfaction, and operational efficiencies that protect your practice’s reputation.
How do cleaning services adapt to seasonal infection surges?
During seasonal surges we increase cleaning and disinfection frequency, intensify high‑touch cleaning, and deploy additional tools like electrostatic spraying where appropriate. Regular communication between facility managers and cleaning providers enables timely schedule and method adjustments so the facility remains compliant and safe for patients and staff.
Conclusion
Choosing specialized medical office cleaning in Ann Arbor ensures a safer, compliant environment that protects patients and staff. With tailored plans, documented protocols, and experienced teams, clinics can lower healthcare‑associated infection risk and strengthen patient confidence. McCoy Maintenance combines local experience with clinical‑grade methods to meet each clinic’s needs. Contact us to discuss a custom cleaning solution for your practice.
